A Decrease in Ph Corresponds to Which of the Following
A increases when body temperature drops so that the skin does not freeze B is controlled mainly by decreasing pH C increases when the environmental temperature rises D is not an important source of nutrients and oxygen for skin cells C Peripheral resistance ________. So the ph Which is equal to negative log 01.
Blood flow will be changed 2 times lesser.

. Students who viewed this also studied. Increase in atomic number. 1 1 2 2 1 4 3 1 3 4 4 2 4 5 2 3.
Which of the following always corresponds to a fall in the short run output A from ECON 203 at Concordia University. C an increase in hydronium ion concentration. B a decrease in hydronium ion concentration.
Saturation m ро 4 Which of the above curves corresponds to myoglobin in a solution containing physiological concentrations of CO2 and BPG at a pH of 7. Which of the above curves corresponds to myoglobin corresponds to a decrease in the pH 68. Answered Sep 16.
A patient with normal left ventricular function who is receiving intravenous dobutamine as part of a diagnostic study for ischemia. Blood flow will be changed 4 times higher. A base has a pH higher than 70.
A decrease in the pH of an aqueous solution corresponds to a An increase of the from CHEMISTRY CHM1045 at Broward College. The ph that corresponds to this concentration is equal to one. Answered Sep 16 2016 by Bred_Dragon.
7 If Curve 3 corresponds to isolated hemoglobin in a solution containing physiological concentrations of CO2 and BPG at a pH of 7 which curve corresponds to the dissociation of hemoglobin into its component subunits. As stated in a above a lowered pH more acidic environment decreases hemoglobins affinity for oxygen. Which of the following statements about a base is true.
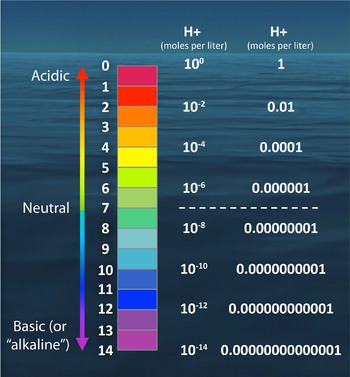
1 Which of the curves depicts the protein with the highest p50. Which of the following pHs corresponds to a highly acidic solution. 2 Which of the curves depicts the protein with lowest oxygen affinity.
As it migrates through a gradient of increasing pH however the proteins overall charge will decrease until the protein reaches the pH region that corresponds to its pI. A normal person whose stroke volume increases as. School University of California Merced.
If its a week for a week for a weak acid means Concentration of H. Now from this law derive if the diameter of the tube increased 4 fold what will be the blood flow. Blood flow will be 256 times higher.
A 53 B 14 C 78 D 92 E 115. Plus is less than 01. Assuming that curve 3 corresponds to isolated haemoglobin Hb placed in a solution containing physiological concentrations of carbon dioxide and 23-BPG at a pH of 74 indicate which of the following curves reflects the following changes in conditions and explain your answer.
A patient in congestive heart failure due to diastolic dysfunction. Which of the following changes in ph corresponds to. E a solution in which H3O 100 10-7 An increase in solution pH corresponds to A a decrease in hydroxide ion concentration.
Pages 42 This preview shows page 30 - 37 out of 42 pages. If Curve 3 corresponds to isolated hemoglobin in a solution containing physiological concentrations of CO2 and BPG at a pH of 7 which curve corresponds to decrease in the concentration of CO2. A increases as blood viscosity increases.
So smaller age plants. Kinetic energy decreases and their potential energy increases. B a decrease in hydronium ion concentration.
Adding a base to an aqueous solution would decrease H. The proteins become focused into sharp stationary bands with each protein positioned at a point in the pH gradient corresponding to its pI. C no change in hydronium ion concentration.
πconstant ΔP change in pressure n viscosity llength r radius. 8 If Curve 3 corresponds to isolated hemoglobin in. Therefore the ph is going to be greater than what.
Increase in atomic number. Decrease in nuclear charge. Asked Sep 16 2016 in Chemistry by Jennifer.
We actually look. High concentrations of CO 2 stabililze the T state. A Increase in CO2 concentration b Decrease in BPG concentration.
HOH- O pH 5 O pH 6 O pH 4. Correct option is. Which of the following reasons corresponds to decrease in IP value from top to bottom in a group.
Course Title CHEM MISC. The standard curve is shifted to the right by an increase in temperature 23-DPG or PCO2 or a decrease in pH. 3 If Curve 3 corresponds to isolated hemoglobin in a solution containing physiological concentrations of CO2 and BPG at a pH of 7 which curve corresponds.
A patient in congestive heart failure. D no change in hydronium ion concentration. D a decrease in.
Which of the following pHs corresponds to a weakly acidic solution. Adding a base to an aqueous solution would decrease the pH. The affinity of hemoglobin for O 2 is diminished by high concentrations of CO 2.
The curve is shifted to the left by the opposite of these conditions. Decrease in screening effect. A rightward shift by definition causes a decrease in the affinity of hemoglobin for oxygen.
Ionization energies increase across a Period from left to right and decrease down a. So this is the information that we can choose before. An increase in solution pH corresponds to A decrease in hydronium ion concentration A buffer solution Closely maintains its original pH A buffer solution does not Does not neutralize only acids Not a salt solution Not a strong base Does not maintain pH at 700 All of these species are involved in the blood buffer system HCO3- CO3 2- H2CO3 CO2.
Therefore C is the correct option. Hemoglobin has less affinity for oxygen. At this point it has no net charge and so it stops moving in the gel.
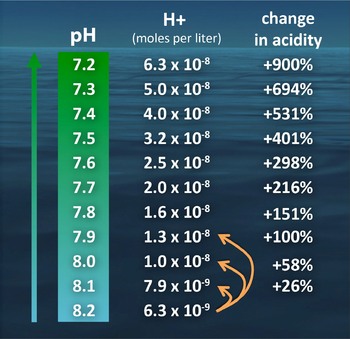
Use the following saturation curves to answer Questions 1-8. Solution for What pH most likely corresponds to the following expression. Which of the following changes in pH corresponds to the greatest percent.
When reactants molecules approaches each other then their kinetic energy changes to potential energy because of increase in repulsion of electron clouds which means that kinetic energy will decrease and potential energy will increase. Curve A corresponds to which of the following. Increase in screening effect.
An increase in solution pH corresponds to A an increase in hydronium ion concentration. There is an increased amount of H in an aqueous solution when a base is added. B a decrease in hydronium ion concentration.

Comments
Post a Comment